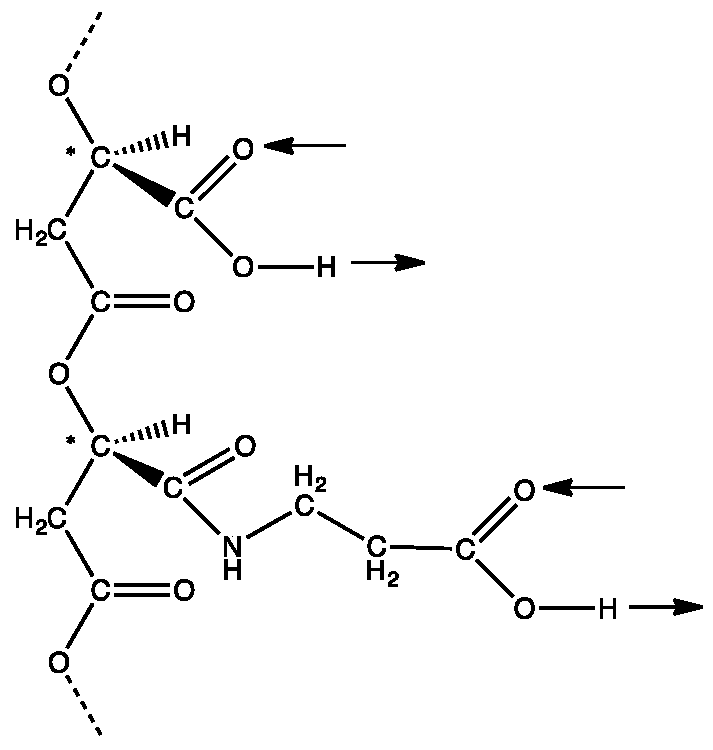
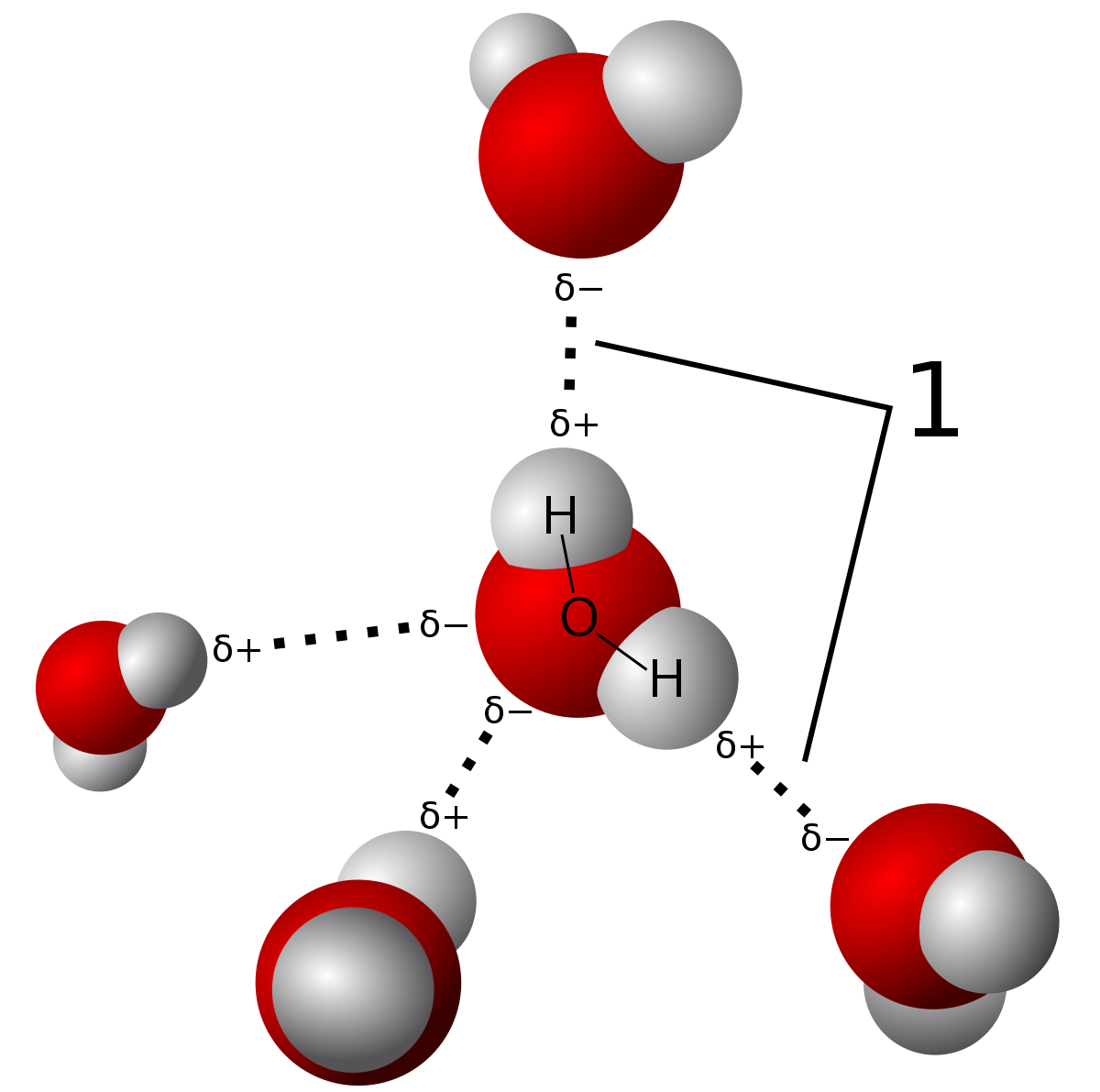
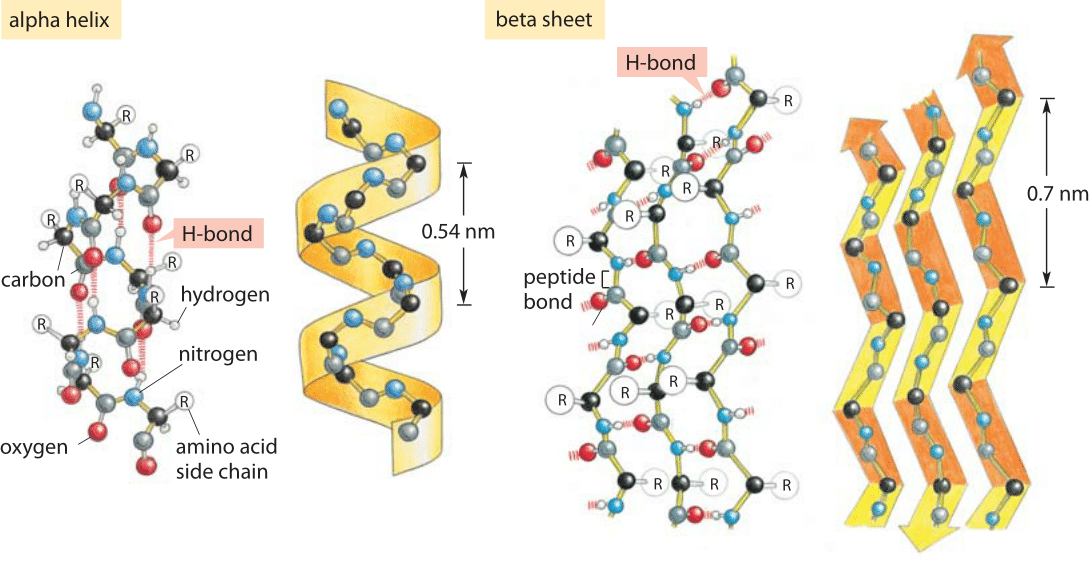
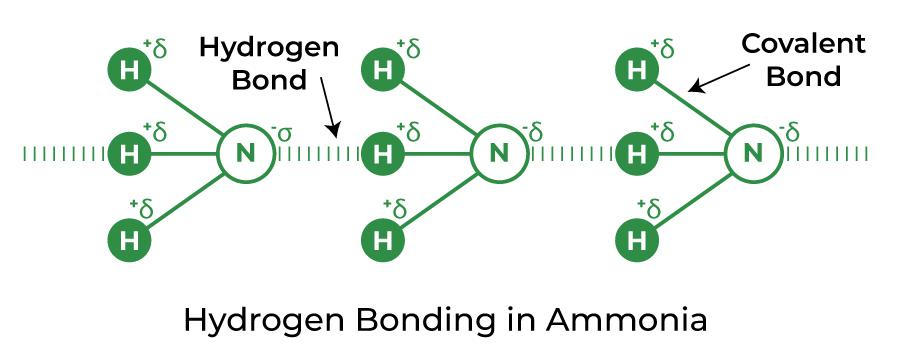
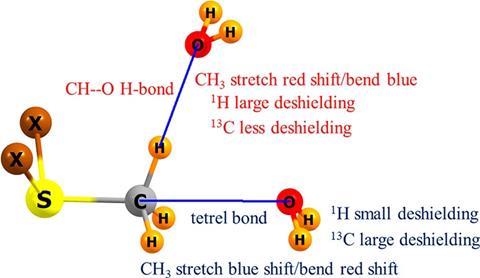
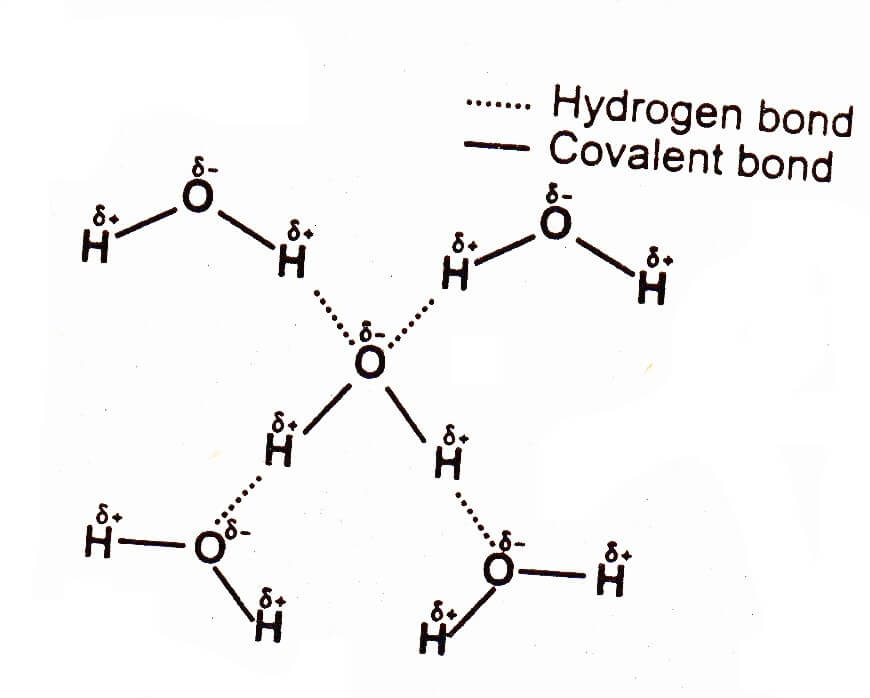
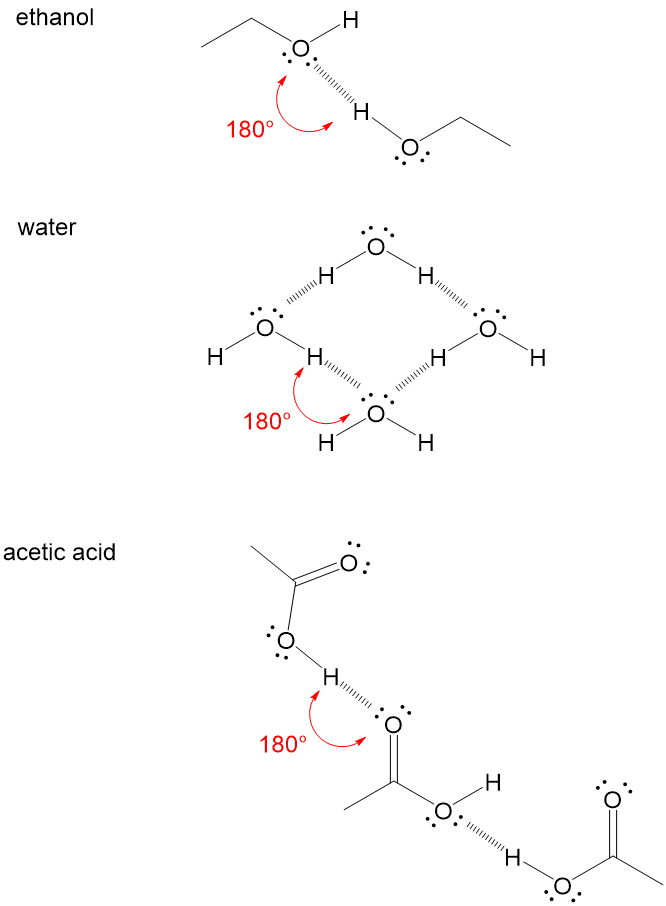
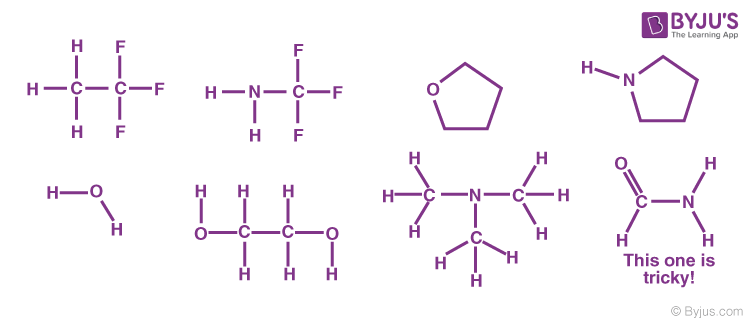
organic chemistry - Why can't alcohols form hydrogen-bonded dimers like carboxylic acids? - Chemistry Stack Exchange

Cooperative Intramolecular Hydrogen Bonding Strongly Enforces cis-Peptoid Folding | Journal of the American Chemical Society
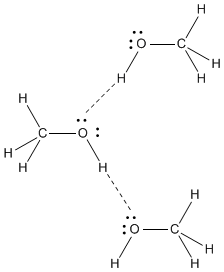
Methanol, CH 4 O (or CH 3 OH, a CH 3 group attached to an OH), is another example of a molecule with a similar molecular weight to ethane. Like formaldehyde, methanol freezes somewhere around-90 o C, but it does not become a gas until it is heated to ...

Coexistence of Intra- and Intermolecular Hydrogen Bonds: Salicylic Acid and Salicylamide and Their Thiol Counterparts | The Journal of Physical Chemistry A

pH-Dependent Hydrogen and Water Binding Energies on Platinum Surfaces as Directly Probed through Surface-Enhanced Infrared Absorption Spectroscopy | Journal of the American Chemical Society